Answer:
2180 g Cu(OH)₂
General Formulas and Concepts:
Chemistry - Atomic Structure
- Reading a Periodic Table
- Using Dimensional Analysis
Step-by-step explanation:
Step 1: Define
22.3 mol Cu(OH)₂
Step 2: Identify Conversions
Molar Mass of Cu - 63.55 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H - 1.01 g/mol
Molar Mass of Cu(OH)₂ - 63.55 + 2(16.00) + 2(1.01) = 97.57 g/mol
Step 3: Convert
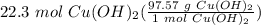 = 2175.81 g Cu(OH)₂
= 2175.81 g Cu(OH)₂
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
2175.81 g Cu(OH)₂ ≈ 2180 g Cu(OH)₂