Answer:
T = 365.58 K
Step-by-step explanation:
Given that,
The concentration of solution, C = 0.750M
Osmotic pressure, P = 22.5 atm
We need to find the temperature of the solution.
The formula for the osmotic pressure is given by :
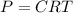
Where
R is gas constant,
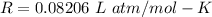
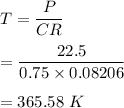
So, the temperature of the solution is 365.58 K.