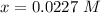Answer:
The value is
Step-by-step explanation:
From the question we are told that
The concentration of
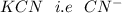 is
is
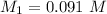
The solubility product constant for
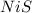 is
is
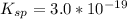
The stability constant for
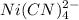 is
is
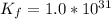
Generally the dissociation reaction for NiS is
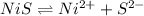
Generally the formation reaction for
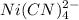 is
is
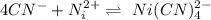
Combining both reaction we have
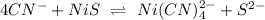
Gnerally the equilibrium constant for this reaction is
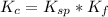
=>
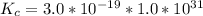
=>
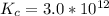
Generally the I C E table for the above reaction is
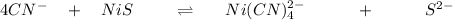
initial [ I] 0.091 0 0
Change [C] -4x +x + x
Equilibrium [E ] 0.091 - 4x x x
Here is x is the amount in term of concentration that is lost by
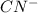 and gained by
and gained by
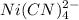 and
and
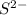
Gnerally the equilibrium constant for this reaction is mathematically represented as
![K_c = ([Ni (CN)_4^(2-) ] [S^(2-) ] )/( [CN^(-)]^4)](https://img.qammunity.org/2021/formulas/chemistry/college/8iprrsrrtint6gdwfq8dndb78ceyw2o2g2.png)
=>
![3.0*10^(12) = (x * x)/( [0.091 - 4x ]^4)](https://img.qammunity.org/2021/formulas/chemistry/college/6acljgm8wb4s6kgzxoz06zemtn1mfeiict.png)
=>
![3.0*10^(12)* [0.091 - 4x ]^4 = x^2](https://img.qammunity.org/2021/formulas/chemistry/college/xyuaiqi9gysh905zeki1clbrg9buf4xb3n.png)
=>
![[0.091 - 4x ]^4 = (x^2)/(3.0*10^(12))](https://img.qammunity.org/2021/formulas/chemistry/college/htcqkau75ivjltwtparhv03ihdy5b8rbtx.png)
=>
![[0.091 - 4x ] = \sqrt[4]{ (x^2)/(3.0*10^(12))}](https://img.qammunity.org/2021/formulas/chemistry/college/aiaat330cyt25di2r6k7sh2pq2mgokkquy.png)
=>
![[0.091 - 4x ] = (√(x) )/(1316)](https://img.qammunity.org/2021/formulas/chemistry/college/7x7jd4fr378h6sr3rjmlye6eoh2vxnwoz2.png)
=>
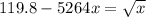
Square both sides
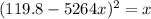
=>
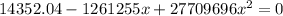
=>
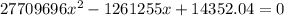
Solving using quadratic equation
The value of x is
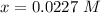
Hence the amount in terms of molarity (concentration) of
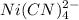 and
and
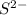 produced at equilibrium is
produced at equilibrium is
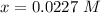 it then means that the amount of NiS (nickel(II) sulfide) lost at equilibrium is
it then means that the amount of NiS (nickel(II) sulfide) lost at equilibrium is
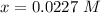
So the molar solubility of nickel(II) sulfide at equilibrium is