Answer:
T₂ = 469.73 K = 196.73 °C
Step-by-step explanation:
First we will find the surface area of rubber stop:
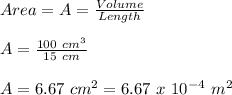
Now, we will find the final pressure required to remove the rubber stop:
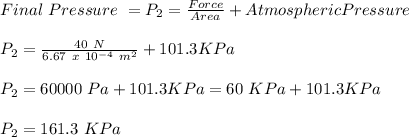
Now, we use equation of state:
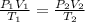
for constant volume due to rigid cylinder:
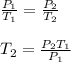
where,
P₁ = initial pressure = 101.3 KPa
P₂ = final pressure = 161.3 KPa
T₁ = Initial Temperature = 22°C = 295 K
T₂ = Final Temperature = ?
Therefore,
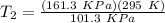
T₂ = 469.73 K = 196.73 °C