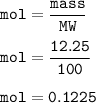Moles of calcium carbonate : 0.1225
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
CaCO₃(s) → CaO(s) + CO₂(g)
mass of CaCO₃ : 12.25 g
mol CaCO₃(MW=100 g/mol) :