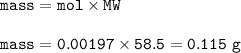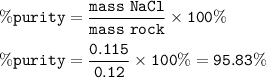moles Cl⁻ : 0.00197
mass NaCl : 0.115
% purity : 95.83%
Further explanation
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
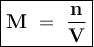
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So to find the number of moles can be expressed as
n = V x M
Reaction
AgNO₃ + NaCl ⇒ AgCl + NaNO₃
Molarity(concentration) of AgNO₃ = 0.1 mol/dm³(L) = 0.1 M
Volume=V of AgNO₃ = 19.7 ml(cm³)
so mol of AgNO₃ :
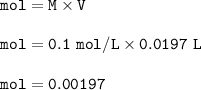 .
.
From the equation, mol ratio AgNO₃ : NaCl = 1 ; 1, so mol NaCl= mol AgNO₃= 0.00197
NaCl⇒Na⁺+Cl⁻
mol Cl⁻ : mol NaCl = 1 : 1, mol Cl⁻ = 0.00197
- mass NaCl (MW=58.5 g/mol) :