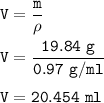Volume of Sodium metal : 20.454 ml
Further explanation
Reaction(balanced) :
2Na + 2H₂O → 2NaOH + H₂
mass NaOH = 34.5 g
mol NaOH(MW=40 g/mol) :
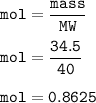
From the equation, mol ratio of Na : NaOH = 2 : 2, so mol Na=mol NaOH=0.8625
Mass Na (Ar=23 g/mol):

Volume Na :