Answer: A) 28%
This value is approximate.
=============================================
Work Shown:
From the periodic table, we are working with these elements
- K = Potassium
- I = Iodine
- Pb = Lead
and they form these relevant compounds
- Potassium iodide = KI
- Lead (ii) iodide =
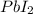
Note: the lead (ii) nitrate chemical formula and it's chemical data (eg: atomic mass) doesn't matter so we won't worry about it.
--------------------
Using the periodic table, specifically the atomic mass of each element mentioned, we can find that:
- Molar Mass of KI = 166.00277 grams per mol
- Molar Mass of PbI2 = 461.00894 grams per mol
They are approximate values based on the average atomic mass.
Those values are handy in calculating the theoretical yield
That theoretical yield is roughly...
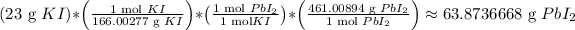
So 23 grams of potassium iodide, and the excess amount of lead (ii) nitrate (the amount of this isn't important as long as it exceeds the potassium iodide amount) react together to produce a theoretical yield of about 63.8736668 grams of lead (ii) iodide precipitate.
-----------------------
Despite us calculating the theoretical yield to be 63.8736668 grams, we actually only got 18 grams. We call this the "actual yield".
To get the percent yield, we divide the actual yield over the theoretical yield and multiply by 100%
So,
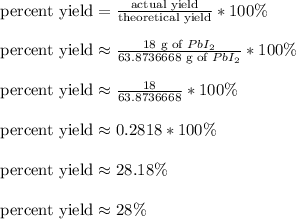
In short, we expected to get a theoretical amount of roughly 63.87 grams of lead (ii) iodide, but instead we got roughly 28% percent of that theoretical amount and got 18 grams of it instead.