An isotope of ₂₀⁴⁰X ⇒₂₀⁴⁴X
Further explanation
The elements in nature have several types of isotopes
Isotopes are atoms that have the same number of protons and a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
In the following element notation,
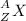
X = symbol of elemental atom
A = mass number
= number of protons + number of neutrons
Z = atomic number
= number of protons = number of electrons, on neutral elements
For symbol of isotope X :
₂₀X⁴⁰ : atomic number = 20, mass number = 40
A. ₄₀²⁰X : atomic number = 40, mass number = 20, neutrons=20
B. ₂₀⁴⁰X²⁺ : atomic number = 20, mass number = 40, neutrons=20
C. ₂₁⁴⁰X : atomic number = 21, mass number = 40, neutrons=19
D. ₂₀⁴⁴X : atomic number = 20, mass number = 44, neutrons=22