Answer:
A)
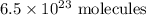
Step-by-step explanation:
m = Mass of water = 38.9
M = Molar mass of water = 18 g/mol
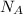 = Avogadro's number =
= Avogadro's number =
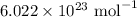
The reaction of electrolysis would be
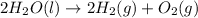
Number of moles of

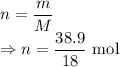
From the reaction it can be seen that 2 moles of
 gives 1 mole of
gives 1 mole of

So, number of moles of
 produced is
produced is
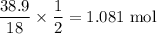
Number of molecules
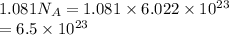
So,
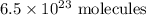 of oxygen is produced.
of oxygen is produced.