Answer:
Step-by-step explanation:
A chemical formula can be defined as a notation that is used to show which element and how many is contained in a chemical compound.
Also, in chemistry, the sum of charges of the anion and the cation of any ionic compound is always equal to zero.
A chemical equation is considered to be balanced when the amount of reactants on the left is equal to the amount of products on the right.
Therefore;
[2]FeBr3 + [3]Na2S → [1]Fe2S3 + [6]NaBr
In the above chemical equation, we will balance the reactants in the chemical equation with the smallest coefficients possible;
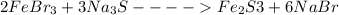
Two (2) moles of Iron (III) Bromide reacts with two (2) moles of Sodium Sulfide to form Iron (III) Sulfide and Sodium Bromide.