The molecular formula : C₄H₈O₂
Further explanation
Maybe a compound is 54.5% carbon, 9.1% hydrogen and 36.4% oxygen,so :
mol C :
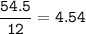
mol H :

mol O :
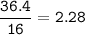
Divide by 2.28(the smallest ratio) :
C H : O =
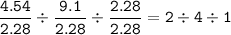
The empirical formula : C₂H₄O
(The empirical formula)n=molecular formula
(2.12+4.1+16)n=88
(44)n=88⇒n=2