Answer:
t = 15825.11 years
Explanation:
From the given information:
We can make use of the activity of the sample present after time "t" to determine the age of the sample.
This can be expressed by the formula:
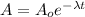 --- (1)
--- (1)
where;
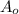 = activity of the sample at time t = 0
= activity of the sample at time t = 0
 = disintegration constant
= disintegration constant
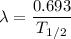
If we replace the value of
 into equation (1), we have:
into equation (1), we have:
![A = A_o e^{ \Bigg [ -{ (0.693)/(T_(1/2)) \Bigg ] } t}](https://img.qammunity.org/2021/formulas/mathematics/college/a77ir05vqvgzdycwpamqea5xpnww6flphc.png)
![(A)/(A_o) = e^{ \Bigg [ -{ (0.693)/(T_(1/2)) \Bigg ] } t}](https://img.qammunity.org/2021/formulas/mathematics/college/xv7yiukx5sx10apaxc8k58p4e1x5axtvnj.png)
By rearrangement:
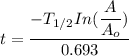
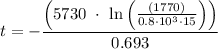
t = 15825.11 years