Answer:
The amount of heat that is released is -925.2 cal
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is the amount of heat that a body can receive or release without affecting its molecular structure, that is, it does not change the state (solid, liquid, gaseous). In other words, sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state.
The equation that allows to calculate heat exchanges is:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the variation in temperature.
In this case:
- c= 1
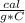
- m= 25.7 g
- ΔT= Tfinal - Tinitial= 49 °C - 85 °C= -36 °C
Replacing:
Q= 1
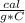 *25.7 g* (-36 C)
*25.7 g* (-36 C)
Solving:
Q= -925.2 cal
The amount of heat that is released is -925.2 cal