Given that,
Mass of magnesium, m = 2 kg
Heat added to it, Q = 8160 J
Increase in temperature,
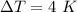
To find,
The specific heat of magnesium.
Solution,
Th formula that is used to find the heat required to raise the temperature in terms of specific heat is given by :
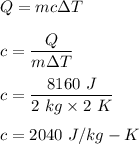
So, the specific heat of magnesium is
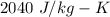 .
.