The given question is incomplete. The complete question is :
Welding is an industrial process that is used to join pieces of metal. A certain mixture of gases used in welding is composed of carbon dioxide and argon. The partial pressure of carbon dioxide is 0.080 atm and the partial pressure of argon is 0.24 atm. What is the total pressure of the mixture.
Answer: 0.32 atm
Step-by-step explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
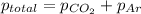
Given :
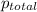 =total pressure of gases = ?
=total pressure of gases = ?
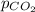 = partial pressure of carbon dioxide = 0.080 atm
= partial pressure of carbon dioxide = 0.080 atm
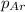 = partial pressure of argon = 0.24 atm
= partial pressure of argon = 0.24 atm
putting in the values we get:
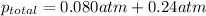
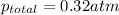
Thus the total pressure of the gases is 0.32 atm