Answer:
28.01 percent
Step-by-step explanation:
Iron (III) sulphate,
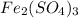 , has 2 atoms of Fe.
, has 2 atoms of Fe.
One atom of Fe has a molar weight of 56. Hence, 2 atoms of Fe would have a total molar weight of 56 x 2 = 112 g/mol
Molar weight of
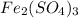 = 399.88 g/mol
= 399.88 g/mol
Percentage Fe in
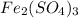 = 112/399.88 x 100%
= 112/399.88 x 100%
= 28.01%
The percentage of iron is iron (III) sulphate is, therefore, 28.01 percent.