Volume (liters) of aqueous 0.325 M nitric acid is 0.132 L
Further explanation
Reaction
2HNO₃ + Ba(OH)₂ → Ba(NO₃)₂ + 2H₂O
mass of Ba(OH)₂ = 3.68 g
mol Ba(OH)₂(MW=171,34 g/mol) :
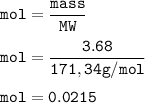
From the equation, mol ratio of HNO₃ : Ba(OH)₂ = 2 : 1, so mol HNO₃:
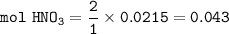
Molarity of HNO₃ = 0.325, then the volume of HNO₃ :
