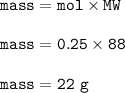Mass of iron sulfide obtained : 22 g
Further explanation
Reaction
Fe+S⇒FeS
8 grams of sulfur and 28 grams of iron are taken for the reaction
mol of Fe(Ar=56 g/mol) :
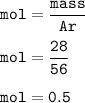
mol of S(Ar=32 g/mol) :
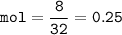
Limiting reactant ⇒ S(smaller mol ratio), excess reactant : Fe
mol of FeS based on limiting (S) ⇒0.25(mol ratio from equation = 1 : 1)
Mass FeS(MW=88 g/mol) :