Answer:
1.67 moles
Step-by-step explanation:
From the balanced equation of reaction:
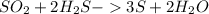
1 mole of sulfur dioxide, SO2, is required to produce 3 moles of sulfur, S.
If 1 mole SO2 = 3 moles S, then, how many moles of SO2 would be required for 5 moles S?
Moles of SO2 needed = 5 x 1/3
= 5/3 or 1.67 moles
Hence, 1.67 moles of SO2 would be required to produce 5.0 moles of S.