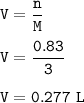The number of liters of 3.00 M lead (II) iodide : 0.277 L
Further explanation
Reaction(balanced)
Pb(NO₃)₂(aq) + 2KI(aq) → 2KNO₃(aq) + PbI₂(s)
moles of KI = 1.66
From the equation, mol ratio of KI : PbI₂ = 2 : 1, so mol PbI₂ :
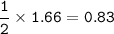
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
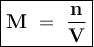
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So the number of liters(V) of 3.00 M lead (II) iodide-PbI₂ (n=0.83, M=3):