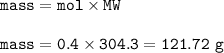The mass of Ba(CIO₃)₂ : 121.72 g
Further explanation
Reaction
Ba(OH)₂ + 2HCIO₃ ----> Ba(CIO₃)₂ + 2H₂O
Limiting reactant :
Ba(OH)₂ : 2HCIO₃ =
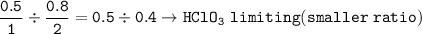
mol Ba(CIO₃)₂ based on limiting reactant(HCIO₃) :
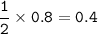
MW Ba(CIO₃)₂ =Ba+2.Cl+6.O=137.3 + 2 x 35.5 + 6 x 16 = 304.3
mass of Ba(CIO₃)₂ :