The correct statement : The atomic number of the radioactive nuclide is 90.
Further explanation
In the following element notation,
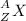
X = symbol of elemental atom
A = mass number
= number of protons + number of neutrons
Z = atomic number
= number of protons = number of electrons, on neutral elements
The symbol for a radioactive nuclide :
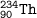
atomic number = 90
mass number = 234
number of neutrons = 234-90=144
number of protons = atomic number = 90