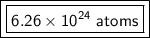
Answer:
Step-by-step explanation:
To convert from moles to atoms, we must use a number called Avogadro's Number which is:
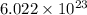
This number tells us the amount of atoms in a mole.
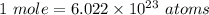
To convert from moles to atoms, we must multiply Avogadro's number by the amount of moles.
We know there are 10.4 moles of Chlorine. Multiply 10.4 (molar amount) by 6.022 * 10²³ (Avogadro's Number)
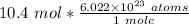

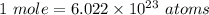
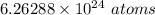
Let's round to 3 significant figures, because the original measurement 10.4, has 3 sig figs (1, 0, and 4)
For the number 6.26288, 3 significant figures is the hundredths place. The 2 in the thousandth place tells us to keep the 6 in the hundredths place.
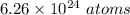
There are about 6.26 × 10²⁴ atoms in 10.4 moles of chlorine.