Answer:
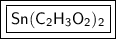
Step-by-step explanation:
First, find what tin and acetate is.
Next, find the charge on acetate, which is -1.
The Roman Numeral 2 in parentheses after tin (II), signifies that the tin has a charge of +2.
We need to balance the charges, so they will equal 0.
The best way to balance is by using 2 acetate molecules
- 2 acetate = -2
- Tin = +2
- -2 +2=0
If we want 2 acetate molecules, we must add a subscript of 2 after acetate.
Add the tin (Sn) in front
- Tin (II) Acetate: Sn (C₂H₃O₂)₂
The formula for tin (II) acetate is Sn (C₂H₃O₂)₂