Answer:
We will need to know Avogadro's number and the molar mass of sucrose for this problem to do dimensional analysis.
- Avogadro's number: 6.022 × 10²³ molecules
- Molar mass of sucrose: 342.2965 g/mol
250g ×
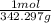 ×
×
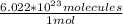 = 4.398 molecules
= 4.398 molecules
There are 4.398 sucrose molecules in 250 grams of sucrose.