Answer:
3.64896 grams Zr
Step-by-step explanation:
Given: amount of moles of Zr: 0.040
Find: the amount of grams of Zr
1. Find molar mass of Zr
- after looking at the periodic table, you can tell it's 91.224 g/mol
2. Solve
(
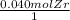 )(
)(
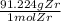 )
)
- the mol units get cancelled out, and you're left with 0.040 x 91.224 g
3. Multiply that out: 3.64896 grams of zirconium