The number of moles of O₂ required for the reaction, given that 12 moles of CuFeS are consumed is 24 moles
How to calculate the number of mole of O₂ required?
First, we shall write the balanced equation for the the reaction involving O₂ and CuFeS. This is shown below:
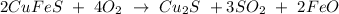
From the above equation,
2 moles of CuFeS required 4 moles of O₂
Finally, we shall calculate the number of moles of O₂ required for the reaction, given that 12 moles of CuFeS are consumed. Details below:
From the above equation,
2 moles of CuFeS required 4 moles of O₂
Therefore,
12 moles of CuFeS will require =
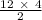 = 24 moles of O₂
= 24 moles of O₂
Thus, we can conclude that the number of moles of O₂ requaired for the reaction is 24 moles
Complete question:
A chemist carried out a reaction between CuFeS and O2. How many moles of O2 are required if 12 moles of CuFeS are consumed?