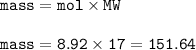The theoretical yield of the reaction : 151.64 g
The limiting reactant : N₂
The excess reactant : H₂
Further explanation
Reaction
N₂+3H₂⇒2NH₃
A method that can be used to find limiting reactants is to divide the number of moles of known substances by their respective coefficients(mol ratio), and the smaller become a limiting reactant
mass of N₂=125 g
mol N₂(MW=28 g/mol) :
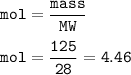
mass H₂ = 125 g
mol H₂(MW= 2 g/mol) :
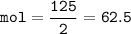
From the equation, mol ratio N₂ : H₂ = 1 : 3
Limiting reactant : N₂ : H₂ =
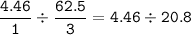
N₂ : limiting reactant
H₂ : excess reactant
mol NH₃ based on limiting(N₂) (from equation N₂ : NH₃ = 1 : 2)
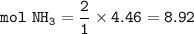
mass NH₃ (MW=17 g/mol) :