Answer:
1.76 x 10²²molecucles
Step-by-step explanation:
Given parameters:
Mass of the sugar C₁₂H₂₂O₁₁ = 10g
Unknown:
Number of molecules = ?
Solution:
The number of molecules can be determined if we find the number of moles first.
Number of moles =

Molar mass of C₁₂H₂₂O₁₁ = 12(12) + 22(1) + 11(16) = 342g/mol
Number of moles =
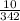 = 0.029mole
= 0.029mole
1 mole of C₁₂H₂₂O₁₁ will contain 6.02 x 10²³
0.029 mole of C₁₂H₂₂O₁₁ will be made up of 0.029 x 6.02 x 10²³
= 1.76 x 10²²molecucles