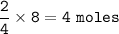Moles of chromium (lll) oxide produced : 4 moles
Further explanation
The reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
4Cr+3O₂⇒2Cr₂O₃
moles of Cr=8 moles
From the equation, mol ratio Cr : Cr₂O₃ = 4 : 2, so mol Cr₂O₃ :