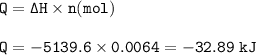The energy produced : -32.89 kJ
Further explanation
Delta H reaction (ΔH) is the amount of heat / heat change between the system and its environment
(ΔH) can be positive (endothermic = requires heat) or negative (exothermic = releasing heat)
The enthalpy of combustion of naphthalene (MW = 128.17 g/mol) is -5139.6 kJ/mol.
For 0.8210 g of naphthalene :
mol =
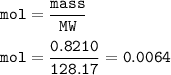
The energy produced :