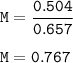The concentration of the HCI solution : 0.767 M
Further explanation
Reaction
Na₂CO₃ (aq) + 2 HCl (aq) → 2 NaCl (aq) + CO₂ (g) + H₂O (l)
mass of CO₂ = 11.1 g
mol of CO₂ (MW= 44,01 g/mol) :
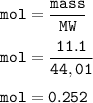
From the equation above, mol ratio of HCl : CO₂ = 2 : 1, so mol HCl :
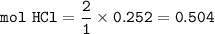
Molarity shows the number of moles of solute in every 1 liter of solution.
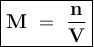
The molarity of unknown HCl :
mol=n=0.504
volume=V=657 ml=0.657 L