The final temperature of the mixture : 21.1° C
Further explanation
The law of conservation of energy can be applied to heat changes, i.e. the heat received / absorbed is the same as the heat released
Q in(gained) = Q out(lost)
Heat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C
∆T = temperature difference, ° C / K
Q ethanol=Q water
mass ethanol=
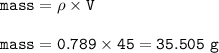
mass water =
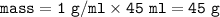
then the heat transfer :
