Answer:
a) 2.01 g
Step-by-step explanation:
- Na₂CO₃ (s) + 2AgNO₃ (aq) → Ag₂CO₃ (s) + 2NaNO₃
First we convert 0.0302 mol AgNO₃ to Na₂CO₃ moles, in order to calculate how many Na₂CO₃ moles reacted:
- 0.0302 mol AgNO₃ *
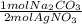 = 0.0151 mol Na₂CO₃
= 0.0151 mol Na₂CO₃
So the remaining Na₂CO₃ moles are:
- 0.0340 - 0.0151 = 0.0189 moles Na₂CO₃
Finally we convert Na₂CO₃ moles into grams, using its molar mass:
- 0.0189 moles Na₂CO₃ * 106 g/mol = 2.003 g Na₂CO₃
The closest answer is option a).