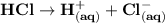Answer:
Ionization
Step-by-step explanation:
Molecular compounds are chemical compounds composed of discrete molecules. A molecular compound undergoes ionization when being dissolved in water and the formation of ions are being produced. For example, hydrogen chloride is a molecular compound, when it dissolves in water, ionization is being carried out, and ions are being formed.