Answer:
The ratio of the new internal energy to the old is 4
Step-by-step explanation:
let the old pressure of the gas = P₁
let the old volume of the gas = V₁
then, the new pressure of the gas = 2P₁
the new volume of the gas = 2V₁
The internal energy of the gas is given as;
PV = nRT
PV = k
P₁V₁ = P₂V₂
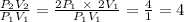
The ratio of the new internal energy to the old is 4