Answer:
v = 1247.92 m/s
Step-by-step explanation:
The formula for kinetic energy is given as follows:
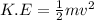
Another formula that is used for Kinetic Energy is given as:
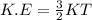
Comparing both formulae for K.E:
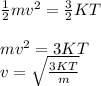
where,
v = rms speed of helium molecule = ?
K = Boltzmann Constant = 1.38 x 10⁻²³ J/k
T = Absolute Temperature = 250 K
m = mass of helium molecule = 6.646 x 10⁻²⁷ kg
Therefore,
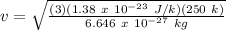
v = 1247.92 m/s