Answer: Two equatorial positions so as to minimize the repulsion.
Step-by-step explanation:
Formula used for calculating number of electrons :
![(1)/(2)[V+N-C+A]](https://img.qammunity.org/2021/formulas/chemistry/college/qhuclhqwb2x84tso011bzrgm05l2evmien.png)
where,
V = number of valence electrons present in central atom = 8
N = number of monovalent atoms bonded to central atom = 4
C = charge of cation = 0
A = charge of anion = 0
Now we have to determine the hybridization of the
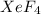 molecule.
molecule.
![(1)/(2)[8+4-0+0]=6](https://img.qammunity.org/2021/formulas/chemistry/college/lgkzonpbhp4t3tanse3o65i5pljmy8shgw.png)
Bond pair electrons = 4
Lone pair electrons = 2
The number of electrons are 6 that means the hybridization will be
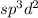 and the electronic geometry of the molecule will be octahedral.
and the electronic geometry of the molecule will be octahedral.
The molecular geometry will be square planar as two alternate positio ns will be occupied by lone pair of electrons so as to minimize the repulsion.