Answer:
76.1%
Step-by-step explanation:
The reaction that takes place is:
- BaCl₂ + K₂SO₄ → BaSO₄ + 2KCl
First we determine how many moles of each reactant were added:
- BaCl₂ ⇒ 80 mL * 0.812 M = 64.96 mmol BaCl₂
- K₂SO₄ ⇒ 40 mL * 1.52 M = 60.8 mmol K₂SO₄
Thus K₂SO₄ is the limiting reactant.
Using the moles of the limiting reactant we calculate how many moles of BaSO₄ would have been produced if the % yield was 100%:
- 60.8 mmol K₂SO₄ *
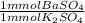 = 60.8 mmol BaSO₄
= 60.8 mmol BaSO₄
Then we convert that theoretical amount into grams, using the molar mass of BaSO₄:
- 60.8 mmol BaSO₄ * 233.38 mg/mmol = 14189.504 mg BaSO₄
- 14189.504 mg BaSO₄ / 1000 = 14.2 g BaSO₄
Finally we calculate the % yield:
- % yield = 10.8 g / 14.2 g * 100 %