Answer:
Elemental gold to have a Face-centered cubic structure.
Step-by-step explanation:
From the information given:
Radius of gold = 144 pm
Its density = 19.32 g/cm³
Assuming the structure is a face-centered cubic structure, we can determine the density of the crystal by using the following:
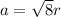
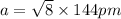
a = 407 pm
In a unit cell, Volume (V) = a³
V = (407 pm)³
V = 6.74 × 10⁷ pm³
V = 6.74 × 10⁻²³ cm³
Recall that:
Net no. of an atom in an FCC unit cell = 4
Thus;
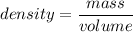
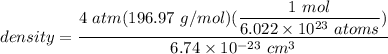
density d = 19.41 g/cm³
Similarly; For a body-centered cubic structure
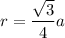
where;
r = 144
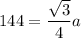
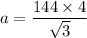
a = 332.56 pm
In a unit cell, Volume V = a³
V = (332.56 pm)³
V = 3.68 × 10⁷ pm³
V 3.68 × 10⁻²³ cm³
Recall that:
Net no. of atoms in BCC cell = 2
∴
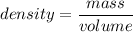
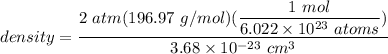
density =17.78 g/cm³
From the two calculate densities, we will realize that the density in the face-centered cubic structure is closer to the given density.
This makes the elemental gold to have a Face-centered cubic structure.