Answer:
58.0 g/mol
Step-by-step explanation:
The reaction that takes place is:
- MCl₂ + 2AgNO₃ → 2AgCl + M(NO₃)₂
First we calculate how many moles of silver chloride were produced, using its molar mass:
- 6.41 g AgCl ÷ 143.32 g/mol = 0.0447 mol AgCl
Then we convert AgCl moles into MCl₂ moles, using the stoichiometric ratio:
- 0.0447 mol AgCl *
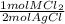 = 0.0224 mol MCl₂
= 0.0224 mol MCl₂
Now we calculate the molar mass of MCl₂, using the original mass of the sample:
- 2.86 g / 0.0224 mol = 127.68 g/mol
We can write the molar mass of MCl₂ as:
- Molar Mass MCl₂ = Molar Mass of M + (Molar Mass of Cl)*2
- 127.68 g/mol = Molar Mass of M + (35.45 g/mol)*2
Finally we calculate the molar mass of M:
- Molar Mass of M = 57 g/mol
The closest option is 58.0 g/mol.