Answer:
W = - 118.24 J (negative sign shows that work is done on piston)
Step-by-step explanation:
First, we find the change in internal energy of the diatomic gas by using the following formula:
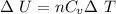
where,
ΔU = Change in internal energy of gas = ?
n = no. of moles of gas = 0.0884 mole
Cv = Molar Specific Heat at constant volume = 5R/2 (for diatomic gases)
Cv = 5(8.314 J/mol.K)/2 = 20.785 J/mol.K
ΔT = Rise in Temperature = 18.8 K
Therefore,
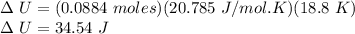
Now, we can apply First Law of Thermodynamics as follows:

where,
ΔQ = Heat flow = - 83.7 J (negative sign due to outflow)
W = Work done = ?
Therefore,
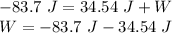
W = - 118.24 J (negative sign shows that work is done on piston)