Answer:
99.3%
Step-by-step explanation:
The percent by mass of the solute can be expressed as:
- % mass =
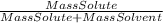 * 100%
* 100%
And for this problem:
- Mass of Solute = Mass of sodium lithium chloride = 29 g
- Mass of Solvent = Mass of Water
So to calculate the percent by mass first we need to calculate the mass of water, to do so we use its density (1 g/L):
- 202 mL is equal to (202/1000) 0.202 L.
Density water = mass water / volume
- 1 g/L = mass water / 0.202 L
Now we have all the data required to calculate the % mass:
- % mass =
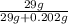 * 100 % = 99.3%
* 100 % = 99.3%