Answer:
V ≈ 646.50 L
General Formulas and Concepts:
Chemistry - Gas Laws
- Reading a Periodic Table
- Stoichiometry
- Combined Gas Law: PV = nRT
- R constant - 62.4 (L · torr)/(mol · K)
- Kelvin Conversion: K = °C + 273.15
Step-by-step explanation:
Step 1: Define
RxN: N₂H₄ (g) + O₂ (g) → N₂ (g) + 2H₂O (l)
Given: 34.9 °C, 755.08 torr, 914.894 g H₂O
Step 2: Identify Conversions
Kelvin Conversion
Molar Mass of H - 1.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H₂O - 2(1.01) + 16.00 = 18.02 g/mol
Step 3: Convert
Stoichiometry:
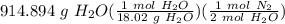 = 25.3955 mol N₂
= 25.3955 mol N₂
Temperature: 34.9 + 273.15 = 308.05 K
Step 4: Find Volume
- Substitute variables: (755.08 torr)V = (25.3955 mol)(62.4 (L · torr)/(mol · K))(308.05 K)
- Multiply: (755.08 torr)V = 488160 L · torr
- Isolate V: V = 646.502 L
Step 5: Check
We are given 5 sig figs as our lowest. Follow sig fig rules and round.
646.502 L ≈ 646.50 L