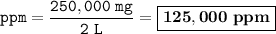The concentration in ppm = 125,000 ppm
Further explanation
The concentration of a substance can be expressed in several quantities such as moles, percent (%) weight / volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
ppm or parts per million (ppm) :
For water solvent = 1 ppm ⇒ 1 mg/L(1 kg=1 L,ρ water=1 kg/L)
In percent mass = 1 ppm ⇒ 1 mg/ 1 kg
Solution contains 0.25 kg of lead in 2 kg of water :
Solvent = water = 2 kg = 2 L
Solute= lead=0.25 kg=250000 mg
So the concentration in ppm :