The monoatomic gas : He
Further explanation
In general, the gas equation can be written
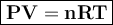
where
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08206 L.atm / mol K
T = temperature, Kelvin
P 380.9 mmHg = 0.5 atm
T 20 C+273 = 293 K
We can find molar mass of gas :
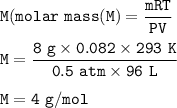
monatomic gas means a single element, usually a noble gas. The noble gas element with mass 4 is He