Mass of this substance = 9.928 g
Further explanation
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
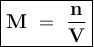
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So to find the number of moles can be expressed as
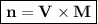
mol of substance -Lithium nitrite - LiNO₂ :
V = 250 ml = 0.25 L
M = 0.75 M
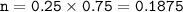
So mass of this substance - LiNO₂ (MW=52,947 g/mol) :
