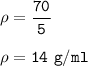The density of the rock in g/mL : 14 g/ml
Complete question :
A rock with a mass of 70 grams is dropped into a graduated cylinder with 30 mL of water in it. The water level rose to 35 mL calculate the density of the rock in g/mL
Further explanation
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
Density formula:
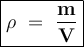
ρ = density
m = mass
v = volume
mass of rock = 70 g
Volume of the rock : 35 - 30 = 5 ml
So the density :