Answer:
13.4mol of Mg
Step-by-step explanation:
Given parameters:
Mass of magnesium = 321g
Unknown:
Number of moles = ?
Solution:
The number of moles of a substance is given as;
Number of moles =

Molar mass of Mg = 24g/mol
Insert the parameters and solve;
Number of moles =
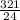 = 13.4mol of Mg
= 13.4mol of Mg